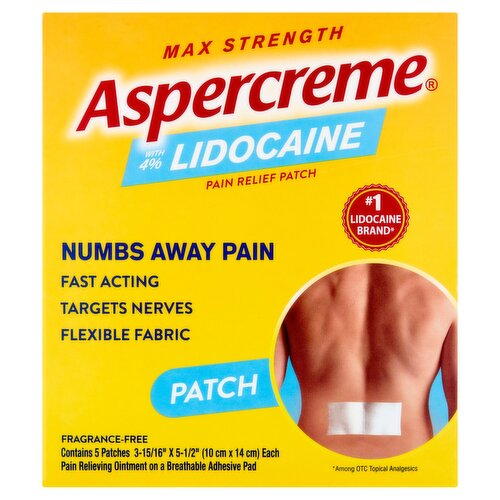
Aspercreme Max Strength with 4% Lidocaine Pain Relief Patch, 5 count
Count
Ingredients
Inactive Ingredients: Aluminum Glycinate, Aluminum Hydroxide, Cellulose Gum, Glycerin, Methyl Acrylate/2-Ethylhexyl Acrylate Copolymer, Methylparaben, Nonoxynol-30, Polyacrylic Acid, Polysorbate 80, Propylene Glycol, Silica, Sodium Polyacrylate, Tartaric Acid, Titanium Dioxide, Urea, WaterBrand Information
#1 Lidocaine Brand* *Among OTC Topical Analgesics
Product Detail
Pain Relieving Ointment on a Breathable Adhesive Pad Drug Facts Active ingredient - Purpose Lidocaine 4% - Topical anesthetic Use For the temporary relief of pain
Preparation & Usage
For easy removal of embossed backing: Pop, Peel, Apply
Features
Numbs Away Pain
Fast Acting
Targets Nerves
Flexible Fabric
Fragrance Free
Targeted No-Mess Relief - Back, Neck, Leg, Arm
Gets your back to your active self fast
Ingredients
Inactive Ingredients: Aluminum Glycinate, Aluminum Hydroxide, Cellulose Gum, Glycerin, Methyl Acrylate/2-Ethylhexyl Acrylate Copolymer, Methylparaben, Nonoxynol-30, Polyacrylic Acid, Polysorbate 80, Propylene Glycol, Silica, Sodium Polyacrylate, Tartaric Acid, Titanium Dioxide, Urea, WaterLegal
ACTUAL PRODUCT PACKAGING AND MATERIALS MAY CONTAIN ADDITIONAL AND/OR DIFFERENT INGREDIENT, NUTRITIONAL OR PROPER USAGE INFORMATION THAN THE INFORMATION DISPLAYED ON OUR WEBSITE. YOU SHOULD NOT RELY SOLELY ON THE INFORMATION DISPLAYED ON OUR WEBSITE AND YOU SHOULD ALWAYS READ LABELS, WARNINGS AND DIRECTIONS PRIOR TO USING OR CONSUMING A PRODUCT. IF YOU HAVE QUESTIONS OR REQUIRE MORE INFORMATION ABOUT A PRODUCT, YOU SHOULD CONTACT THE MANUFACTURER DIRECTLY. CONTENT ON THIS WEBSITE IS FOR GENERAL REFERENCE PURPOSES ONLY AND IS NOT INTENDED TO SUBSTITUTE FOR ADVICE GIVEN BY A PHYSICIAN, PHARMACIST OR OTHER LICENSED HEALTH CARE PROFESSIONAL. YOU SHOULD NOT USE THE INFORMATION PRESENTED ON THIS WEBSITE FOR SELF-DIAGNOSIS OR FOR TREATING A HEALTH PROBLEM. WAKEFERN FOOD CORP. AND ITS SERVICE PROVIDERS ASSUME NO LIABILITY FOR INACCURACIES OR MISSTATEMENTS REGARDING ANY PRODUCT.
